Understanding the Ocean's Role in Carbon Storage and Emissions
Written on
Chapter 1: The Ocean's Carbon Reservoir
The ocean serves as a significant carbon reservoir, containing approximately 50 times the carbon found in the atmosphere. The latest estimates from the Global Carbon Budget indicate that the ocean holds around 38,700 gigatons (Gton) of carbon, while the atmosphere contains about 860 Gton. To illustrate, adding just 1 Gton of carbon to the atmosphere raises the carbon dioxide (CO₂) concentration by roughly 0.5 ppm (parts-per-million).
In this article, we will delve into why the ocean maintains such a vast amount of carbon compared to the atmosphere and discuss the fate of this carbon as we strive to cut down emissions.
This paragraph will result in an indented block of text, typically used for quoting other text.
Section 1.1: Tracking Atmospheric Carbon
Human activities have led to a rise in carbon dioxide levels both in the atmosphere and the ocean. This phenomenon is explained by Henry’s law, which states that the amount of CO₂ dissolved in water correlates with the concentration of CO₂ in the air above it. Essentially, as atmospheric CO₂ levels increase, so do those in the ocean.
In Hawaii, researchers have been monitoring CO₂ levels in both the air and surface waters. The findings reveal that oceanic CO₂ concentrations are closely following those in the atmosphere, indicating that the ocean effectively tracks atmospheric changes.
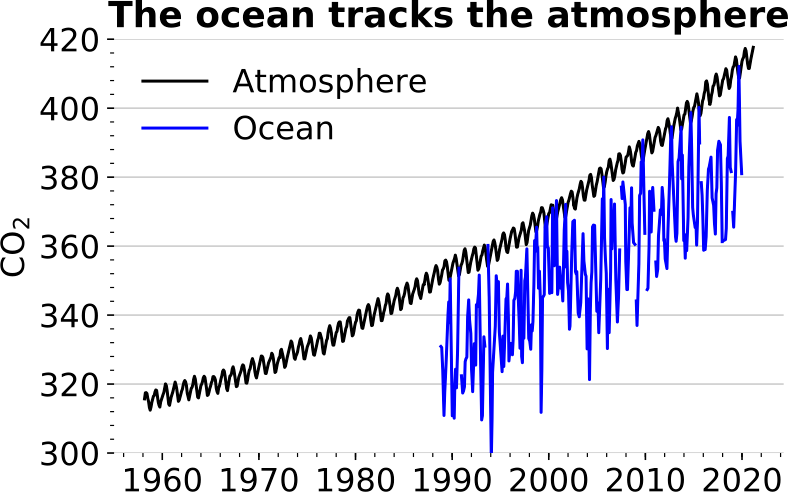
Section 1.2: The Chemistry of Ocean Carbonation
The process of carbonation occurs when atmospheric CO₂ dissolves in ocean water, creating carbonic acid (H₂CO₃). This acid subsequently breaks down into hydrogen, bicarbonate (HCO₃⁻), and carbonate ions (CO₃²⁻). The increased hydrogen ions lower the ocean's pH, contributing to ocean acidification, often referred to as the "other CO₂ problem." This is a familiar concept, as it parallels the carbonation found in beverages like soda.
The collective concentration of H₂CO₃, CO₃²⁻, and HCO₃⁻ is termed dissolved inorganic carbon (DIC), with its final concentrations influenced by the water's pH. Approximately 98% of the ocean's carbon resides within this DIC pool, enhancing the ocean's capacity to absorb more CO₂ from the atmosphere. This mechanism explains the vast disparity in carbon levels between the ocean and the atmosphere.
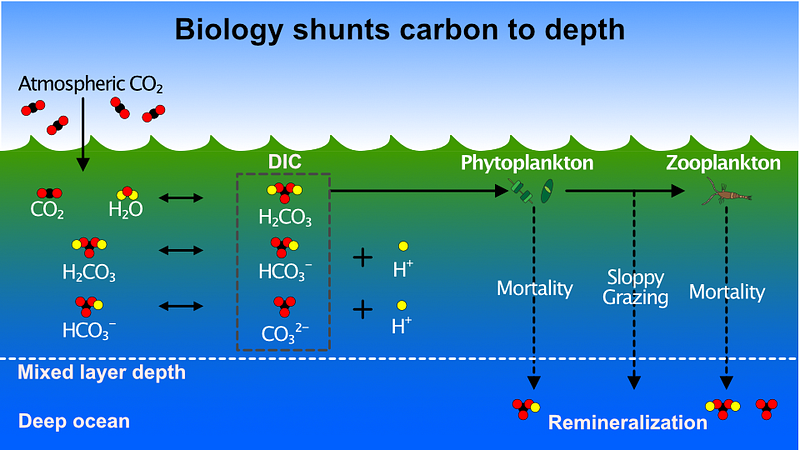
Chapter 2: Implications of Reducing Emissions
The first video, "Ocean Carbon Dioxide Levels: An Invisible Time Bomb?" explores the potential dangers of rising CO₂ levels in the ocean and their implications for climate change.
As we begin to significantly lower our emissions, the ocean, while currently acting as a carbon sink, may transition into a source of carbon for the atmosphere. Eventually, a threshold will be reached where CO₂ concentrations in the ocean exceed those in the air, resulting in carbon being released back into the atmosphere. This process occurs due to diffusion, where gases naturally move from areas of high concentration to low concentration.
The ocean's gradual release of accumulated carbon should be anticipated, emphasizing that its role as a carbon sink is not permanent. While it currently aids in mitigating climate change, it could hinder our future efforts if emissions are not curtailed swiftly. The sooner we act on reducing emissions, the less adverse impact the ocean will have on our climate strategies moving forward.
The second video, "An Ocean of Climate Solutions" by Peter de Menocal at TEDxBoston, discusses innovative solutions to tackle climate change, highlighting the ocean's critical role.
Special thanks to Rachana Maniyar, Ph.D., for reviewing drafts of this article. Thank you for your continued support of Medium writers.