Understanding the Phases of Matter and Their Impact on Climate
Written on
Chapter 1: Introduction to Matter
In this discussion, we'll explore some foundational concepts. Before diving into the essential elements of our climate system—such as the atmosphere, oceans, and cryosphere (ice)—it’s important to revisit the states of matter and specifically, water.
There are four recognized phases of matter, but our focus will be on the first three: solids, liquids, and gases, while plasma is excluded from our primary concerns. Among these, liquids, gases, and plasma are categorized as fluids due to their ability to have molecules that move freely and are not restricted in place. Solids possess the least amount of molecular energy, followed by liquids, then gases, and finally plasma. Transitioning from a lower energy state (for example, from solid to liquid or liquid to gas) necessitates a significant input of heat, whereas changing from a higher energy state to a lower one (like gas to liquid) results in heat release.
Section 1.1: Solids and Their Characteristics
Solids are materials where atoms are held in fixed positions relative to one another. Interestingly, what we identify as solid matter is primarily composed of empty space, with the exception of extraordinarily dense objects like neutron stars. The atoms in solids do not move freely; rather, they are held in place by varying strengths of chemical bonds. Among all matter phases, solids exhibit the least molecular energy.
Subsection 1.1.1: Liquids Defined
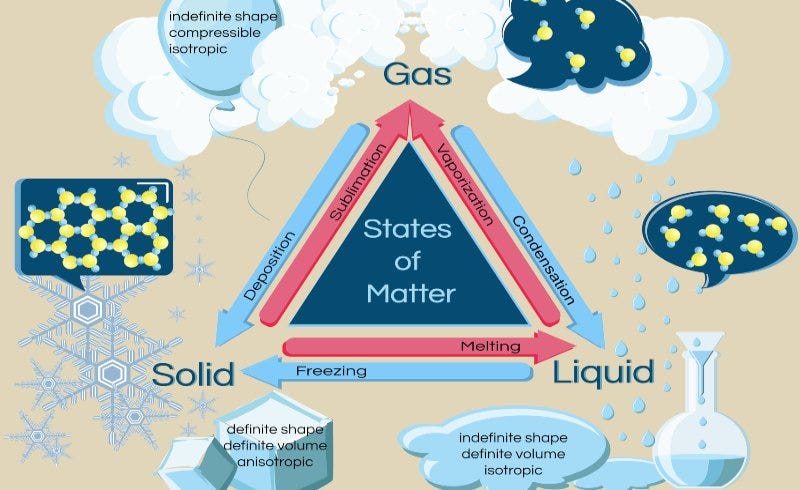
In contrast, a liquid is characterized by molecules that can move with relative freedom. Weak chemical bonds maintain a consistent distance between these molecules, allowing liquids to adapt their shape to fit their containers—whether that container is a glass or the floor, resulting in a puddle—while their volume remains unchanged.
Section 1.2: Gases and Their Behavior
Gases are fluids where molecules have nearly complete freedom of movement, traveling in any direction dictated by their momentum. Gases will expand to occupy any available space, as their molecular paths alter only upon colliding with one another. According to Newton’s second law, an object in motion will stay in motion unless an external force intervenes.
The gravitational force also plays a role in retaining gases close to Earth, particularly those of adequate density. It is believed that Earth has lost a considerable amount of atmospheric hydrogen to space, as H2 molecules rise and escape due to their light weight. Similar to a small-scale rocket, these hydrogen molecules attain escape velocity and drift off into the cosmos, albeit slowly.
Chapter 2: The Role of Water in Climate Understanding
In the Huberman Lab Podcast #97, Dr. Layne Norton discusses the intricate relationship between health, fat loss, and lean muscle. This dialogue emphasizes how understanding biological processes—like those of water—can enrich our comprehension of the climate's fundamental dynamics.
Tomorrow, we will delve into the fascinating world of water molecules, revealing insights you might not have anticipated.
Stay healthy!
Read more from The Good Men Project on Medium:
What the Coronavirus Can Teach Us All About Sex, Love, and Survival
Navigating the complexities of our reality can often feel overwhelming.
5 Signs It Isn’t Love, Even Though They Say, ‘I Love You’
Thomas Fiffer highlights five red flags that often go unnoticed in relationships.
Never Marry a Man With These 14 Traits
Identifying these traits can be crucial for a happy relationship.
The original piece was published on The Good Men Project.
About Michael Sutherland: A dedicated family man, scientist, singer, writer, dancer, and an enthusiastic lover of life.